Warren L. Lee
Associate Professor
MD, University of Toronto, 1997
PhD, University of Toronto, 2006
Postdoc, Weill Medical College of Cornell University, 2007
| Address | St. Michael's Hospital LKS, Room 613 Toronto, ON M5B 1W8 |
| Lab | Lee Lab |
| Lab Phone | 4168646060 |
| Office Phone | 416-864-6060-77656 |
| warren.lee@unityhealth.to |
Dr. Lee received his M.D. from the University of Toronto and was awarded the Cody Gold Medal. He completed residencies in Internal Medicine, Respirology, and Critical Care Medicine in Toronto. He then undertook research training in the Program in Cell Biology at the Hospital for Sick Children, completing a PhD in 2006 in the lab of Sergio Grinstein. This was followed by postdoctoral training in Microbiology and Immunology at Weill Medical College of Cornell University in the lab of Carl F. Nathan (New York, NY). His research focuses on the mechanisms of endothelial permeability, with specific interest in both endothelial LDL transcytosis and in paracellular leakage during lung injury/sepsis.
In the News
Research Lab
The lab is a collegial and enthusiastic environment. Students participate in weekly lab meeting and journal club and acquire analytical and presentation skills. There is also a weekly joint lab meeting among all the labs on the floor, giving the student the opportunity to present their work to (and learn from) a multidisciplinary audience.
Learn more: Lee Lab
Research Description
Mechanisms of endothelial permeability
We are an endothelial biology lab with a focus on permeability. Every blood vessel in the body is lined with a specialized layer of polarized cells known as endothelium. An essential function of the endothelial monolayer is the regulation of barrier integrity, which prevents the leakage of plasma and proteins out of the circulation while still permitting the flux of nutrients and immune cells to target tissues.
In principle, permeability of the endothelial monolayer can reflect contributions from leaking between endothelial cells (paracellular leak) and through individual endothelial cells (transcellular leak, or transcytosis). It is widely accepted that paracellular leak predominates during inflammatory states such as sepsis and acute lung injury. Accordingly, by far the majority of research on endothelial permeability has focused on this route of endothelial permeability: the methods of study are relatively straight-forward and there is obvious relevance to human disease. In contrast, the contribution of transcytosis to overall endothelial permeability is relatively obscure, particularly in the setting of inflammation. This is largely due to technical difficulties in distinguishing transcellular permeability from intercellular gaps, particularly in a dynamic and quantifiable way. In addition, endothelial cells grown in culture appear to lose the ability to perform transcytosis as they are passaged. Much of the initial work on transcytosis used electron microscopy of animal tissues, an expensive and often a mostly descriptive endeavour. Transcytosis (at least in the apical to basal direction) is best described for the plasma protein albumin and is mediated by caveolae, small vesicles that bud off from the apical endothelial surface and release their cargo at the basal membrane. This process requires the protein caveolin-1 and the large GTPase dynamin; the latter is thought to mediate the scission of internalized caveolae from the apical plasmalemma.
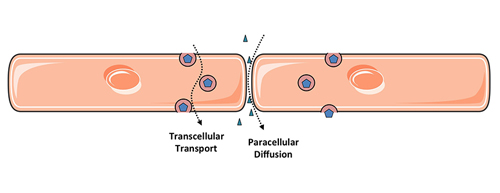
Figure 1. Two routes of endothelial permeability
My lab is interested in both routes of endothelial permeability and how they are related.
We study paracellular leak during inflammation, using acute lung injury induced by the human influenza A virus as a model system. We investigate how the virus induces lung endothelial permeability to cause pulmonary edema, a characteristic clinical feature of severe influenza infections in humans. We have reported effects of the virus on lung endothelial viability and on tight junction integrity; interestingly, at least some of the effect of the virus on endothelial barrier integrity is independent of viral replication and involves degradation of the tight junction constituent claudin-5. Remarkably, restoration of endothelial barrier integrity using a Tie2 agonist peptide does not impair viral clearance and is sufficient to improve survival from otherwise fatal influenza in a pre-clinical model. This suggests that optimization of the host response to influenza (e.g. strengthening vascular integrity) represents a viable and unappreciated therapeutic approach and this is now a major focus of the lab. We are also broadly interested in enhancing delivery of drugs and genes to the injured lung.
Enhancing lung endothelial barrier integrity as a novel therapy for severe influenza infections
Another area of study in the lab is the contribution of endothelial transcytosis to the overall permeability of the endothelium to macromolecules. Interestingly, endothelial transcytosis underlies the first stage of atherosclerosis. Accumulation of LDL-derived cholesterol under the arterial endothelium triggers an inflammatory reaction that culminates in luminal narrowing and eventually an unstable arterial plaque. However, how the LDL gets under the endothelium is poorly understood. Autopsy studies on young individuals dying of non-cardiac causes reveal a healthy, continuous endothelial layer overlying cholesterol deposits and the average LDL particle is too large to pass through intact cell-cell junctions; thus, LDL is likely to cross the endothelium by transcytosis. The canonical model of LDL receptor-initiated endocytosis does not explain LDL accumulation in the arterial intima. We devised an assay to quantify LDL transcytosis by individual cells in a confluent monolayer using total internal reflection fluorescence microscopy, complimented by traditional transwell assays and an ex vivo perfusion assay. The TIRF assay in particular is well suited to mechanistic studies. Using this approach, we were the first to report an unexpected role for the scavenger receptor SR-BI in LDL transcytosis (Armstrong et al., Cardiovascular Research 2015). We are now actively investigating the regulation of LDL transcytosis.
The work in the lab is centred on live cell imaging that is complemented by traditional biochemical and molecular biology approaches. The lab is located in the Keenan Research Centre for Biomedical Science, St. Michael’s Hospital (a tertiary care teaching hospital). Dr. Lee and the hospital are affiliated both with the University of Toronto and with Ryerson University.
Awards & Distinctions
2018 — 3rd Prize, Global Health Care Innovation Academy (Hong Kong) Juried research competition
2016 — Canada Research Chair, Mechanisms of Endothelial Permeability
2016-2017 — The Lung Association – Pfizer Research Award
2011-2016 — Early Researcher Award, Government of Ontario
Courses Taught
BCH2120 Studies of tissue barriers: Regulation of phenotype and transport across the epithelium and endothelium
BCH473Y Advanced Research Project in Biochemistry
Publications
View all publications on PubMed
Endothelial HMGB1 is a critical regulator of LDL transcytosis via a SREBP2-SR-BI axis
Ghaffari S, Jang E, Naderinabi F, Sanwal R, Khosraviani N, Wang C, Steinberg B, Goldenberg N, Ikeda J, Lee WL
Arteriosclerosis, Thrombosis, and Vascular Biology 2020 (in press). PMID 33054399 Read
The endothelial barrier is not rate-limiting to insulin action in the myocardium of male mice.
Sanwal R, Khosraviani N, Advani SL, Advani A, Lee WL.
Endocrinology. 2020 Feb 27;doi: 10.1210/endocr/bqaa029. [Epub ahead of print] Read
Staphylococcus aureus leukocidins target endothelial DARC to cause lethality in mice
Lubkin A., Lee W.L.*, Alonzo F. 3rd, Wang C., Aligo J., Keller M., Girgis N. M., Reyes-Robles T., Chan R., O’Malley A., Buckley P., Vozhilla N., Vasquez M., Su J., Sugiyama M., Yeung S.T., Coffre M., Bajwa S., Chen E., Martin P., Kim S.Y., Loomis C., Worthen G.S., Shopsin B., Khanna K.M., Weinstock D., Lynch A.S., Koralov S.B., Loke P., Cadwell K., and Torres V.J.* (*co-corresponding authors)
Cell Host Microbe 2019;25:1-8. Read
CD36 mediates albumin transcytosis by dermal but not lung microvascular endothelial cells - role in fatty acid delivery
Raheel H*, Ghaffari S*, Khosraviani N, Mintsopoulos V, Auyeung D, Wang C, Kim YH, Mullen B, Sung H-K, Ho M, Fairn G, Neculai D, Febbraio M, Heit B, Lee WL.
American Journal of Physiology - Lung Cellular and Molecular Physiology 2019 (in press) Read
Lung Ultrasound and Microbubbles Enhance Aminoglycoside Efficacy and Delivery to the Lung in Escherichia coli-induced Pneumonia and Acute Respiratory Distress Syndrome.
Sugiyama MG, Mintsopoulos V, Raheel H, Goldenberg NM, Batt JE, Brochard L, Kuebler WM, Leong-Poi H, Karshafian R, Lee WL.
Am J Respir Crit Care Med. 2018 Aug 1;198(3):404-408. doi: 10.1164/rccm.201711-2259LE. Read
Estrogen inhibits LDL transcytosis by human coronary artery endothelial cells via GPER and SR-BI
Ghaffari S, Naderi Nabi F, Sugiyama MG, Lee WL.
Arteriosclerosis, Thrombosis, and Vascular Biology 2018;38:2283-2294 Read
SR-BI Mediated Transcytosis of HDL in Brain Microvascular Endothelial Cells Is Independent of Caveolin, Clathrin, and PDZK1.
Fung KY, Wang C, Nyegaard S, Heit B, Fairn GD, Lee WL
Frontiers in Physiology. 2017 Oct 30;8:841. Read
A novel assay uncovers an unexpected role for SR-BI in LDL transcytosis.
Armstrong S, Sugiyama M, Fung YY, Gao Y, Wang C, Levy AS, Azizi P, Roufaiel M, Zhu S-N, Neculai D, Yin C, Bolz S-S, Seidah N, Cybulsky M, Heit B, Lee WL.
Cardiovascular Research 2015 Nov 1;108(2):268-77. Read
The Tie2-agonist Vasculotide rescues mice from influenza virus infection
Sugiyama MG, Armstrong SM, Wang C, Hwang D, Leong-Poi H, Advani A, Advani S, Szaszi K, Tabuchi A, Kuebler WM, Van Slyke P, Dumont DJ, Lee WL
Scientific Reports 2015| 5:11030 | DOI: 10.1038/srep11030 Read
Influenza primes human lung microvascular endothelium to leak upon exposure to Staphylococcus aureus
Wang C*, Armstrong SM*, Sugiyama MG, Tabuchi A, Krauszman A, Kuebler WM, Mullen B, Advani S, Advani A, Lee WL
American Journal of Respiratory Cell and Molecular Biology (2015), in press.
Broken barriers: a new take on sepsis pathogenesis.
Goldenberg NM, Steinberg BE, Slutsky AS, Lee WL
Science Translational Medicine 2011, 3(88), 88ps25



